Myth: “Keep the temps below 70 and your terpenes will be fine”
This thinking is super common among drying and curing professionals, but it could possibly ruin your cannabis, because temperature is only one of the two main factors that control evaporation.
Think about this scenario…
Imagine you store your flowers in a Turkey Bag in an open building through the winter. Since it’s winter you think the cool temperatures will protect your terpenes.
However, here in my home area of Southern Oregon, the fall and winter humidity levels frequently get as low as 35% relative humidity.
That’s some seriously dry air!
Cannabis flowers are very porous plant matter that will either absorb or emit moisture until the material equalizes with the Relative Humidity in the outside air within a couple days.
This will happen a little slower inside a Turkey bag because these bags are “resistant” to moisture transfer, but they are by no means water “proof.” So within only a couple days at 35% RH, your buds will equalize to 35% RH.
35% RH inside the buds means your water activity is 0.35 aw. That’s dry enough that the buds will crumble and fall apart under the slightest pressure or rough handling.
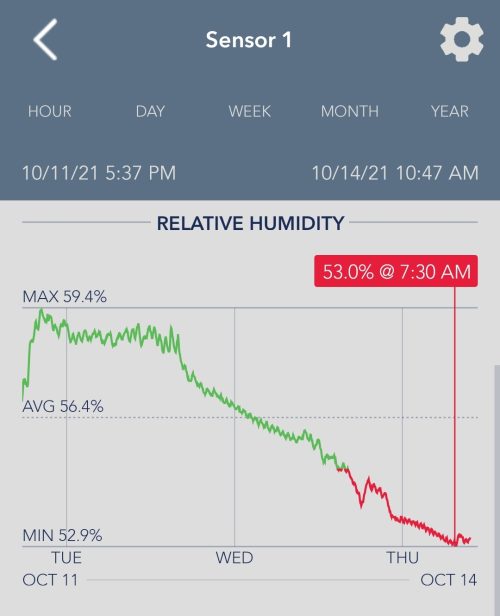
The Turkey Bag Test
This graph shows how cannabis flowers in a sealed Turkey bag dropped from 60% RH to 50% RH in only 3 days. In this case the outside RH was at about 35% so this is a very extreme case.
If you tried the same experiment in an area with an RH of 75%, then the RH in the Turkey bag would have risen.
The difference between the inside and the outside RH determines how fast the RH inside the bag changes.
For example: if the RH inside the bag is 65% and the RH outside the bag is 60% the RH inside the bag will change, but it will be at a very slow rate.
You might be tempted to think, “If I can rehydrate any bud no matter how dry it is, I can just pop a Boveda moisture pack in the bag and bring it back up to a normal moisture” and that would be true! You absolutely can re-hydrate flower material no matter how dry you let it get. I’ve tried it on some scrap buds I didn’t want and it absolutely works with no noticeable effects to the FLOWER material.
But what about the terpenes?
Terpenes are effected by all the same forces of evaporation as water, and some even evaporate faster than water. So while you can absolutely re-hydrate your buds, you can’t ever get those terpenes back if you manage to evaporate them.
What is Evaporation
Evaporation is controlled by two forces that compete against each other.
The first force is from the Atmospheric Pressure which is simply the weight that pushes down on top of us from all the gasses piled above us in the atmosphere. Those gasses do have weight, and that weight acts AGAINST evaporation by applying pressure all around a liquid that keeps the molecules in the liquid from escaping.
The second force is Vapor Pressure which is the force molecules in the substance exert as they try to escape out from the main mass of the substance. All things have a vapor pressure, even solids.
The difference between these two forces (Vapor Pressure and Atmospheric Pressure) determines the speed of evaporation. When the two pressures are far apart, evaporation takes place rapidly. When the two pressures are close together, evaporation slows down.
Temperature affects the Vapor Pressure because temperature is really just a way of describing how fast the molecules in a substance are moving around.
Faster motion = higher temperature.
When the molecules move faster, they can escape from the mass of the material more easily. This means that higher temperatures increase the Vapor pressure, which translates into faster evaporation.
How to Stop Evaporation in a Container
You can’t stop evaporation of an object that is exposed to the elements. However, by using a sealed container with very little free air space, you leave nowhere for your terpenes to evaporate to! They’re trapped! Inside the container a small amount will evaporate, but then eventually the “headspace” or the empty space inside the container will become saturated and no more will evaporate beyond that point.
How to Stop Evaporation in the Drying Room
If you do it right, your drying room will also be a sealed container – just bigger.
In this case, there will be a ton of empty space so you can’t use quite the same strategy.
Here you’ll be trying to maintain a low temperature which reduces the speed of evaporation, in combination with a medium-high humidity which fills the space outside the cannabis with water vapor. The water vapor molecules create a crowded atmosphere outside the cannabis which makes it harder for any molecules in the cannabis (either water or terpenes) to evaporate out.
The balancing act between temperature and humidity is the key part of the curing process that allows enzymes to continue breaking down starches in the plant matter over a long period of time. This enzymatic action will stop completely if you get it too cold or too hot. Around 69F is the sweet spot where you reduce evaporation, but still allow enzymes to continue working.
Perfect parameters for your drying room are:
- 69F temperature
- Low airflow that does not impact the plant matter directly
- RH of 70% in the beginning to middle time, and the 65% for the final week before packaging up.
- Package in moisture proof mylar plastic bags, glass jars, or metal cans with very little air space.
- If the product is not going directly to a customer that same month, place the sealed packages in a freezer for long term storage.